Caco3 2hcl cacl2 h2co3.
Marble reacting with hydrochloric acid equation.
Powdered marble reacts with hydrochloric acid to release bubbles of carbon dioxide gas.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
Calcium carbonate is dissolved by hydrochloric acid thereby forming gaseous carbon dioxide.
Marble reaction with hydrochloric acid.
The combined reactants have a higher chemical potential than the combined products i e.
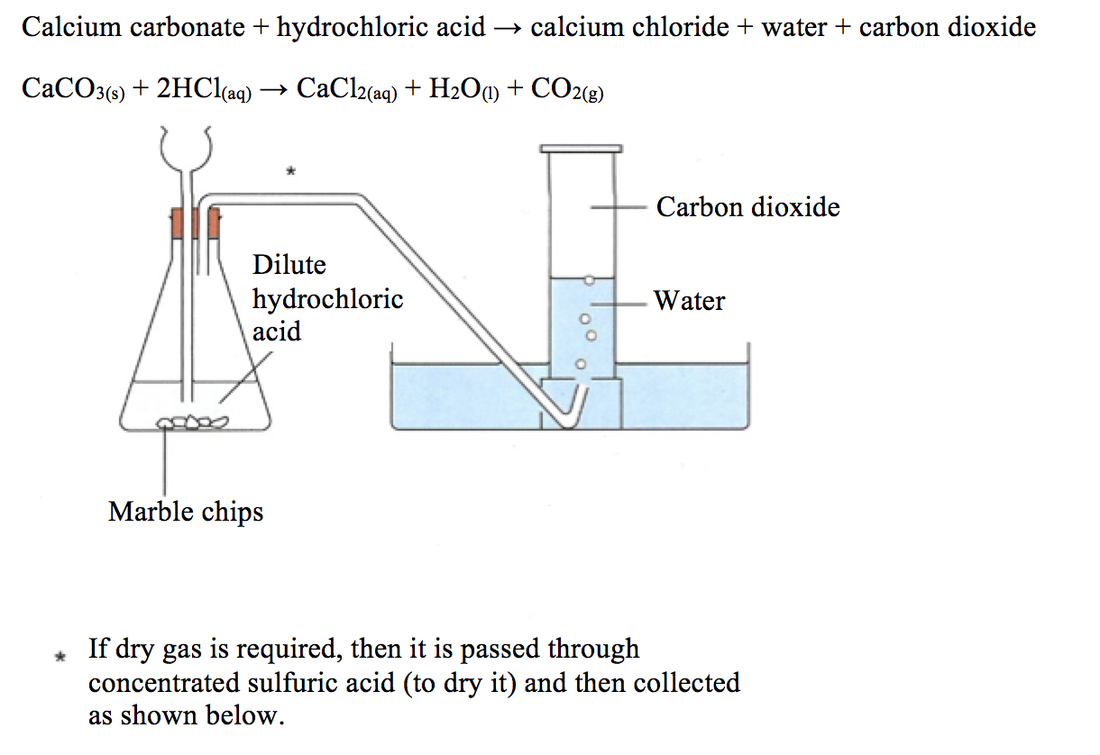
In words calcium carbonate reacts with hydrochloric acid to form calcium chloride water and carbon dioxide.
Marble is mostly made up of calcium carbonate which is caco3.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
Caco3 2hcl cacl2 h2o co2.
Acid rain is one of the top degradation agents for marble artefacts around the world.
H2co3 decomposes easily into h2o and co2 so the equation is.
Pieces of marble are thrown into hydro chloric acid.
Click each image to see positive and negative results of the acid test.
Acids carbonates salts carbon dioxide water marble is caco3 2hcl caco3 cacl2 co2 h2o.
Marble is especially sensitive to the degrading by acidic chemicals also to weathering.
Drop a small amount of dilute hydrochloric acid on an area of your sample that has been scratched by a nail.
Hydrochloric acid 20ml 0 5m 1m 2m marble chips 2g per test large measuring cylinder plastic bowl 3 4 full of water rubber tubing glass conical flask stopwatch method.
Hydrochloric acid is hcl.
I marble chips are made from calcium carbonate hydrochloric acid calcium carbonate calcium chloride water carbon dioxideii the rate of reaction can be increased by increasing the concentration of hydrochloric acid which is the limiting factor in this equation.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details.
Stone surface material may be lost all over or only in spots that are more reactive.
Acid rain contains carbonic acid nitric acid and sulphuric acid co2 no2 and so2.
The reaction takes place spontaneously.
The chemical equation that is going to be followed throughout the experiment will be.